Since up to 60% of preclinical studies cannot be reproduced, are highly biased towards positive results, and often over-interpret small effect sizes we need to place the bar higher to identify the strongest candidate drugs for repurposing. We believe that a combination of systemic reviews (evaluating multiple organs, pathways, or systems within the body at the same time), preclinical randomised confirmatory trials (pRCTs), meta-analyses (statistical cross-examination of a large number of publications on the same topic), and use of big data tools and open access platforms (e.g. pre-clinicaltrials.org) is the most effective approach to identify promising candidate drugs for mechanism-based repurposing and to reduce experimentation on animals later on.
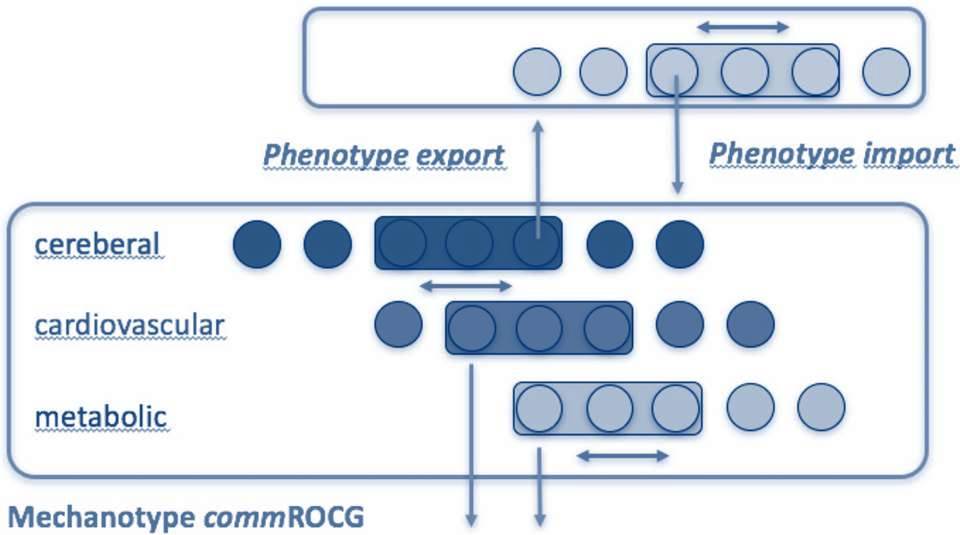
The expression “in silico drug repurposing” alludes to the use of silicon in semiconductor computer chips, but is really just a fancy expression for using computer software and specified algorithms to process a large amount of data and structure it in the most useful way. We use it to identify mechanistically related drug targets in a systems-based approach, including organs or regions of the body that may differ entirely from the initial indication or drug purpose. Eventually, we will match an updated and improved diseasome with the in silico-explored drugome to develop a “virtual patient cohort”. The image below illustrates how we can, for example, use the symptom-phenotype of stroke patients, compare it at the molecular (mechanistic) level with other diseases like heart disease or metabolic diseases, to receive a common in silico-predicted mechanotype.
For the scope of this study, we primarily focus on patients with cerebro-cardiovascular indications, such as stroke, myocardial infarction, or resistant hypertension. In any case, a positive outcome for the patient is always our primary concern. This means that assessment of the respective drug’s effectiveness will happen in a timely manner, and that the experimental drug (versus placebo) will always be given on top of standard therapy. Of course, patients of the placebo versus the experimental group will be matched for age, gender and various relevant co-morbidities. The principles and tools developed during the duration of the multinational REPO-TRIAL project will likely be applicable to many other areas of biomedical research. We also plan to provide an education module for the R&D community.