This section keeps you updated on any major developments throughout the course of the REPO-TRIAL project.
Our publishable summary of the 5th Research Period (1st August 2023 – 31st January 2024) now online. All REPO-TRIAL members were working hard to make the project successful. Click here to read about the final outcomes of the project!
The REPO-TRIAL project officially comes to an end. We are happy to see what has been achieved!
You want to learn more about the REPO-TRIAL project and its outcomes? Click here to read more about the project or watch our REPO-TRIAL Outcomes – Video Series!
Lean back, relax, and enjoy some free education and insight into our Systems Medicine and Drug Repurposing approach by watching our “REPO-TRIAL Outcomes – Video Series” – a row of lectures by the scientific coordinator, work package leaders, and other lead scientists. The videos were recorded in the final months of the project (November 2023 – January 2024) to convey the outcomes of REPO-TRIAL to trainees and the general public, and to ensure a long-lasting educational and societal impact of the project.

Today, our 4th reporting has been successfully submitted. Read our publishable summary of the 4th Research Period (1st February 2022 – 31st July 2023) now online.
REPO-TRIAL is now entering the fifth and final research period. All REPO-TRIAL members are working hard to deliver their final tasks and make the project successful. Stay tuned to read about the final outcomes of the project soon!
Learn more about “Patent Possibilities in Drug Repurposing” by watching this lecture by patent attorney Kolja Adamczyk (NLO – European Patent and Trademark Attorneys) who remotely joined the “RePo4EU National Dissemination Event for Germany” that took place in Munich, Germany, on 3rd May 2023. The online presentation was recorded and later published for training and education within the REPO-TRIAL project.
REPO-HFPEF II, a Phase IIa clinical study in patients with heart failure with preserved ejection fraction, has finalized enrolling its last patient. The trial, which investigates a triple combination of three drugs approved for other therapeutic uses (the pulmonary hypertension drug, vericiguat; the amino acid, L-citrulline; and the vitamin, folic acid) on top of standard therapy, includes now 21 patients. REPO-HFPEF II is part of REPO-TRIAL, a 5.5-year project funded by a Horizon 2020 research grant (No. 777111) from the European Commission. Read the entire press release.
We are exited to share the news that the European Commission has approved our request for a 6-month project extension until January 2024. All REPO-TRIAL members are working hard to complete their final tasks and make the project a success. Stay tuned and read about the outcomes of research period 4 soon!

REPO-TRIAL COMMENCES ITS HEART FAILURE DRUG REPURPOSING TRIAL
REPO-TRIAL has randomised the first patients in REPO-HFpEF II, a Phase IIa clinical trial investigating the treatment of a type of chronic heart failure characterised by stiffness of the left ventricle. HFpEF is common in the elderly and diabetics and has no survival-prolonging treatment. Read the entire press release.
Read our final press release that summarises REPO-TRIAL’s main goals, the clinical studies, and major achievements of the 5.5-year-long project. A big thank you to the whole team of researchers, principal investigators, students, postdocs, clinicians, managers, and meeting organisers who made it all happen!

“REPO-TRIAL prepares the ground to disrupt treatment of ischemic stroke and heart failure with preserved ejection fraction,” stated REPO-TRIAL project coordinator Professor Harald Schmidt, chair of the Department of Pharmacology and Personalised Medicine at Maastricht University in the Netherlands. “We have employed advanced bioinformatics and systems medicine to identify suitable triple combinations of repurposed drugs and advance them into Phase IIa clinical trials for these deadly conditions, with minimal requirements for animal experiments. While both studies are still enrolling patients, and no interim results can be disclosed yet, we are confident that they will break new ground in pathway-based medicine.” Read the entire press release.
As REPO-TRIAL is nearing conclusion, the entire team is currently convening in Madrid for its final General Assembly (GA) Meeting. This closing meeting is the occasion to evaluate results from two ongoing Phase IIa clinical trials, REPO-STROKE II and REPO-HFpEF II. Based on new insights into systems medicine gained at the University of Maastricht, a bioinformatics team distributed between Syddansk Universitiy (Kopenhagen), the Technical University of Munich and Hamburg University in Germany, as well as Newcastle University (United Kingdom) collaborated to design triple combinations of approved drugs that block critical pathways in conditions with significant unmet medical need.

REPO-STROKE II, a Phase IIa clinical study in patients with acute ischemic stroke, has enrolled its first patient. The trial investigates a triple combination of three drugs approved for other therapeutic uses on top of standard therapy. In this context the consortium published a press release with the topic ‘REPO-STROKE II, THE THREE-COMPONENT DRUG REPURPOSING STUDY, STARTS INCLUSION OF PATIENTS‘. Read more.
REPO-TRIAL is getting ready to initiate REPO-STROKE, the first of its two Phase Ib/IIa investigator-initiated clinical trials investigating synergistic combinations of repurposed drugs. In this context the consortium published a press release with the topic ‘REPO-TRIAL PREPARES FOR ITS CLINICAL STUDY IN STROKE PATIENTS‘. Read more.
We would like to welcome Professor Sheila Martins (Universidade Federal do Rio Grande do Sul, Brazil and President-Elect, World Stroke Organization) as new members of the Scientific and Ethical Advisory Board (SEAB).
The following renowned experts from the relevant scientific fields of REPO-TRIAL have also agreed to be part of the REPO-TRIAL Scientific and Ethical Advisory Board:
- Tony Bartlett, Tacit Bio Innovation Limited, United Kingdom
- Johannes Boltze, Professor of Neuroscience, School of Life Sciences, The University of Warwick, United Kingdom
- Emre Guney, Dr, CTO, Head of Discovery and Data Science, STALICLA, Spain
- Thomas Krahn, Professor Dr, Advisor, INVICOL GmbH, Germany
- Carsten Tschöpe, Professor of Medicine and Cardiology, Vice Director of the Dept. of Cardiology, Charité, Germany
The REPO-TRIAL consortium welcomes two new partners
Since 1st February 2021 two new partners support the REPO-TRIAL consortium with achieving their goals. The Martin-Luther-Universität Halle-Wittenberg, one of the oldest Universities in Germany, with Prof. Dr. Daniel Sedding as team leader will be leading the REPO-HFpEF study. And Prof. Dr. Jan Baumbach moved from the Technical University of Munich (TUM) to the University of Hamburg. He will continue leading Work Package 1. Welcome to the REPO-TRIAL team!
The team, led by researchers from Maastricht University (UM), has discovered one of the causes of primary hypertension or high blood pressure and that this cause could be treated with existing and already registered drugs.
Please click on the links below to read the interviews with REPO-TRIAL Coordinator Prof. Harald H.H.W. Schmidt.
- Researchers discover important cause of high blood pressure [Maastricht University | Article in English]
- Onderzoekers ontdekken belangrijke oorzaak van hoge bloeddruk [Maastricht University | Article in Dutch]
- Bestaand geneesmiddel kan groot deel van hoge bloeddruk-patiënten helpen [De Telegraaf | Article in Dutch]
- Onderzoekers UM ontdekken oorzaak hoge bloeddruk [1Limburg | Article in Dutch]
- Oorzaak van hoge bloeddruk ontdekt door internationaal onderzoek [Dokters van Morgen | Article in Dutch]
REPO-TRIAL Coordinator Prof. Harald H.H.W. Schmidt recently gave a talk about “Vom Ende der Medizin wie wir sie kennen” at the Lions Club Niederrhein to give a better overview of the project to the general public.
Dr Ana I. Casas, member of the H2020 project REPO-TRIAL consortium, receives 1M € grant from the Corona-Stiftung program for stroke research.
Her research project NEURONET aims to improve the early detection, prevention and treatment of strokes with the help of a systems medical overall view of the different organs.
Read more [article in German]
New publication “COVID-19 and the Drug Repurposing Tsunami” from Dr. Hermann Mucke, REPO-TRIAL WP4 leader, published in ASSAY and Drug Development Technologies.
Identification of drug repurposing candidates for treatment of Covid-19
In order to find out which existing drugs might be suitable for the treatment of Covid-19, numerous research groups from all over the world are working on systems medicine approaches. A research team from the Chair of Experimental Bioinformatics (ExBio) at the TUM School of Life Sciences of the Technical University of Munich (TUM) has now developed the first online data analysis platform (CoVex) for this purpose:
The Coronavirus Explorer (CoVex) integrates the virus-human interactome for SARS-CoV-2 and SARS-CoV. CoVex is designed to help provide a comprehensive understanding of the infection mechanisms and focuses not only on the virus and its direct interaction partners, but in particular involves the host-protein interaction network.
Just watch the new video on YouTube:
Conference canceled due to COVID-19! –Final COST action OpenMultiMed Conference on Multiscale Systems and Network Medicine
Our main objective is to gather a critical mass of researchers and stakeholders from different disciplines and coordinate these researchers as a team to define, investigate important S&T challenges in multiscale systems medicine and to improve existing and develop novel solutions for multiscale systems medicine.
International Conference on Systems and Network Medicine
18 – 19 March 2020
TranslaTUM, Technischen Universität München
Einsteinstraße 25, 81675 Munich, Germany
Dr. Ana I Casas, one of the WP leader of the REPO-TRIAL consortium, recently gave a talk at the 51st Brazilian Congress of Pharmacology and Experimental Therapeutics (SBFTE) in Maceió, State of Alagoas, the Northeast Region of Brazil. The scientific program covered a wide range of topics in the frontier of pharmacological knowledge. This year the theme of the Meeting was Challenging frontiers in Pharmacology.

REPO-TRIAL Coordinator Prof. Harald H.H.W. Schmidt and Jan Baumbach, member of the REPO-TRIAL consortium were keynote speakers at the First International Conference in Systems and Network Medicine: Applications of Systems Science and Thinking to Biomedicine (Georgetown University, 11-13 September 2019). The conference aims to create an active engagement between participants, encourage future interdisciplinary research opportunities, create collaboration amongst the international systems thinking stakeholders, and foster networking.

4th SC Meeting held in Luxembourg: The REPO-TRIAL consortium was meeting in Luxembourg to discuss the overall progress and first emerging results!

Check out our brand new project video on YouTube!
REPO-TRIAL’s scientific coordinator, clinician, and pharmacologist Prof. Dr. Harald Schmidt, the bioinformatics expert and co-coordinator Prof. Dr. Jan Baumbach, the regulatory expert Dr. Hermann Mucke, and Priv.-Doz. Dr. Benedikt Frank, the neurologist overseeing the stroke study, explain the details of the project during an interview session prior to the 2nd GA Meeting in Vienna, Austria, on March 4th, 2019. We also prepared a German version of the video.
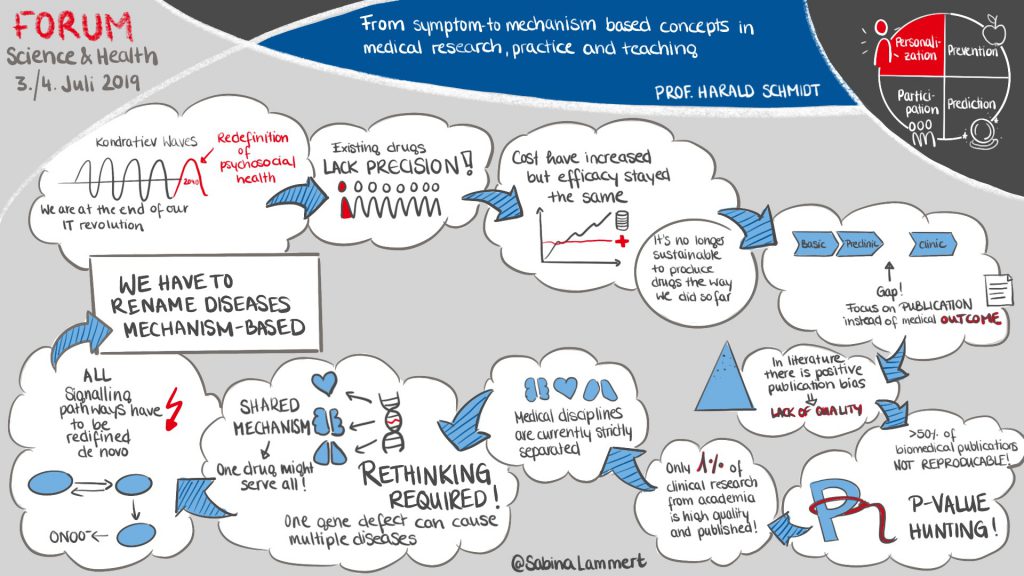
REPO-TRIAL Coordinator Prof. Harald H.H.W. Schmidt talked about “Symptom- to mechanism-based concepts in medical research, practice and teaching” at the forum ‘Science & Health’ in Fürstenfeldbruck, Germany.
Existing drugs fail to provide benefit for most patients. The efficacy of drug discovery is in a constant decline. This poor translational success of biomedical research is due to false incentives, lack of quality/reproducibility and publication bias. The most important reason, however, is our current concept of disease, i.e. mostly by organ or symptom, not by a mechanism. Systems Medicine will lead to a mechanism-based redefinition of disease, precision diagnosis, and precision therapy – eliminating the need for drug discovery, and completely reorganizing how we teach, train and practice medicine.
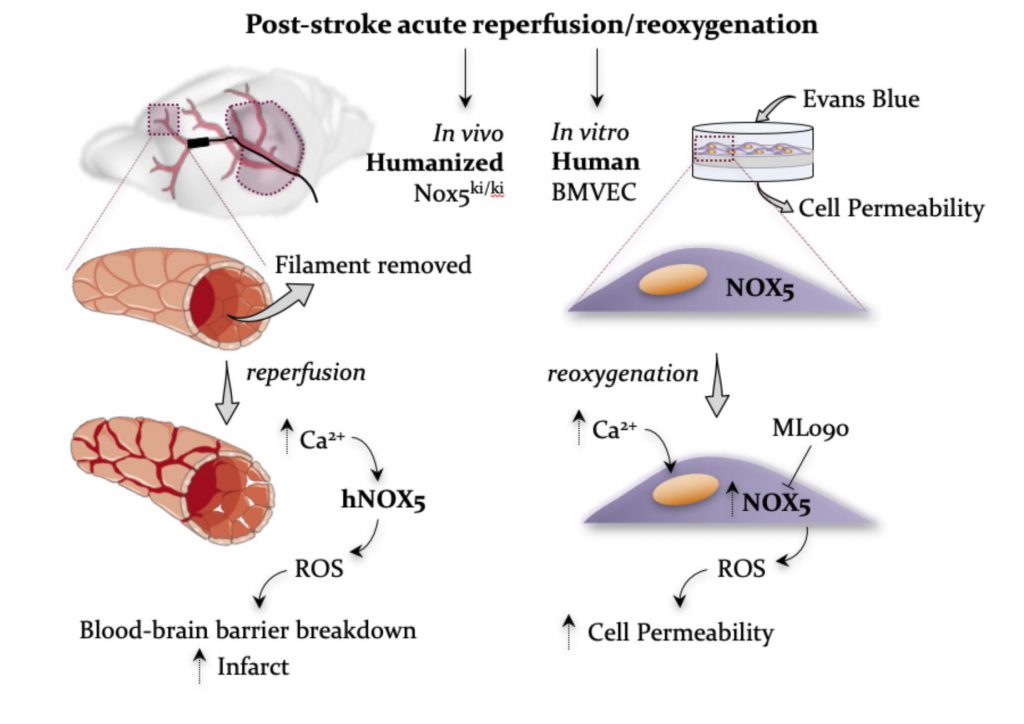
A new study by Dr. Kleinschnitz, Prof. Dr. Harald Schmidt and REPO-TRIAL colleagues with the title “Calcium-dependent blood-brain barrier breakdown by NOX5 limits postreperfusion benefit in stroke” has recently been published in the Journal of Clinical Investigation! In May, this study has been covered in form of a separate article in the news section of the Ärzteblatt (renowned German magazine for medical doctors). Congratulations!
2nd GA Meeting held in Vienna: The EU-funded project is now in its 2nd year & brings experts from systems biology, biochemistry, in silico modelling, neurology and cardiology to one table to tackle complex, mechanistically related diseases! From 4-6 March, the REPO-TRIAL group met in Vienna to discuss the next steps of the project.

REPO-TRIAL Coordinator Dr. Harald H.H.W. Schmidt gave a talk at the World Government Summit in Dubai. View the full presentation on “The End of Medicine as we know it”on YouTube!
E. coli gene regulatory networks are inconsistent with gene expression data
This publication, co-authored by Profs. Harald Schmidt and Jan Baumbach and to be published in Nucleic Acids Research, challenges established knowledge of the systemic biology on the cellular level, finding no system-wide correlation between the expression of transcription factors and their target genes in E. coli. More sophisticated network models are needed, with higher complexity and more dynamic features, to truly understand how gene expression is regulated. The preprint version of the paper can be accessed here.

Harald Schmidt and Jan Baumbach were keynote speakers at the International Congress on Precision Medicine Beyond Cancer.
- Harald Schmidt – ‘The end of medicine as we know it’
- Jan Baumbach – ‘Systems Medicine – or – What I Learned About Arnold Schwarzenegger While Studying Breast Cancer Survival’
This September Coordinator Professor Dr. Harald Schmidt will give a talk on “Disease Cluster-based Drug Repurposing” at the International Conference on Network Medicine and Big Data in Rome
We have barely started our 5-year project and are already busy promoting our science! Ana Casas Guijarro (UM) and Hermann Mucke will hold presentations at the 7th Drug Repositioning, Repurposing and Rescue Conference in Chicago.
# June 27, 2018 9:00 a.m.
Hermann Mucke, H.M. Pharma Consultancy: A Systems Medicine Approach to Drug Repurposing (an introduction to REPO-TRIAL design and objectives)
# June 27, 2018 9:30 a.m.
Ana Casas Guijarro, Dept. of Pharmacology and Personalised Medicine at Maastricht University: Big Data-based Drug Repurposing Strategy: Special Focus on Brain Ischemia (drilling down on the in silico approach for drug repurposing in stroke)
The REPO-TRIAL Kick-off Meeting took place in Innsbruck (Austria), 20-23 February 2018, and was a full success! A big thanks to all attendees!

Four different advisory boards, consisting of experts for each topic, will ensure scientific quality, efficient data management and data analysis, as well as adherence to ethical standards, clinical trial regulations, and diversity requirements.

